Electron Shell Sodium Electron Configuration Bohr Model, PNG
Metal Ion Non-Metals Non-metals - Tend to have 4, 5, 6, or 7 electrons in their outer orbits (shells). They gain electrons to form negative ions (anions) They gain electrons, thus they have the same electron arrangement as the Noble gas in the same row. Try to make a Bohr-Rutherford ion for phosphorous. 15 31 P 3-
.PNG)
Bohr Models and Lewis Dot Diagrams Presentation Chemistry
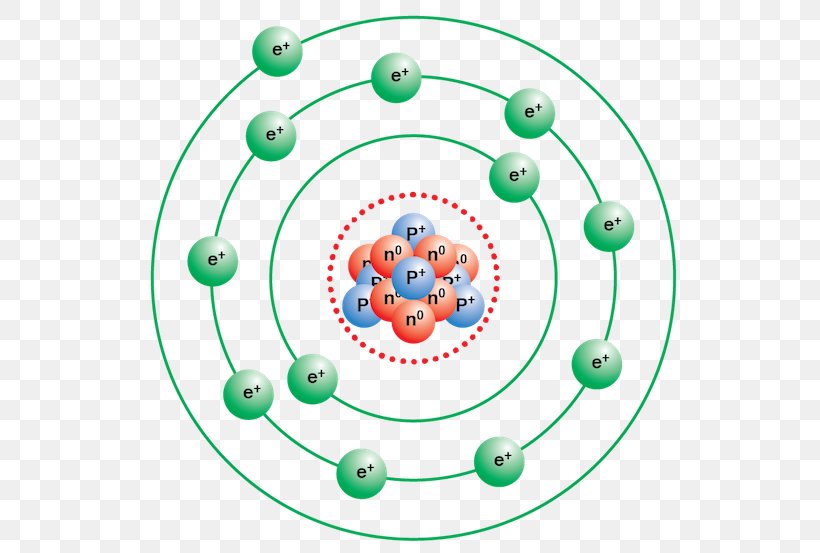
Bohr diagram or Bohr rutherford diagram describes the visual representation of orbiting electrons around the small nucleus. It used different electron shells such as K, L, M, N…so on.

Bohr Diagram Of Sodium Atom model, Atom diagram, Atom model project
In 1913, a Danish physicist, Niels Bohr (1885-1962; Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Bohr's model required only one assumption: The electron moves around the nucleus in circular orbits that can have only certain allowed radii.

Bohr Rutherford Diagram For Sodium
Example 21.4.1: Emitted Photon. According to Bohr's theory two of the allowed orbits in the hydrogen atom have radii of 52.918 and 211.67 pm. Calculate the energy, the frequency, and the wavelength of the photon emitted when the electron moves from the outer to the inner of these two orbits.

Bohr Rutherford Diagram For Sodium
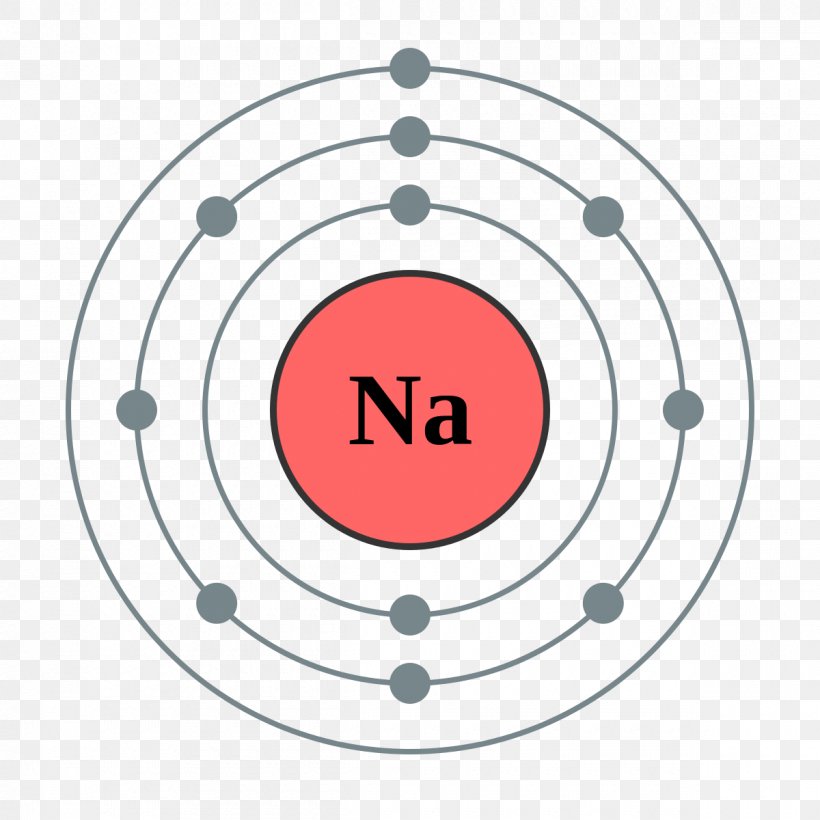
The sodium Bohr Rutherford diagram is a visual representation that helps us understand the atomic structure of sodium. It is based on the Bohr model of the atom and the observations of Ernest Rutherford. This diagram shows the arrangement of electrons in the energy levels or shells around the nucleus of a sodium atom.
Bohr Model Sodium Atom Chemistry Rutherford Model, PNG, 550x553px, Bohr
Figure \(\PageIndex{1}\): A Bhor's model can be used to diagram the location of electrons in each energy shell for an atom. Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Image from Wikimedia commons. Example \(\PageIndex{2}\) Draw the Bohr's model for sodium (Na).

[DIAGRAM] Board Diagram Of Sodium
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.

Bohr Diagram Of Sodium
(a) Draw the Bohr-Rutherford diagram (without neutrons) for an atom of each of the following elements: lithium, oxygen, calcium; and phosphorus. (b) Draw the; Bohr-Rutherford diagram (without neutrons) for the ion formed by each of the elements in (a). (c) Write the chemical symbol for each ion.
Sodium Bohr Diagram
Bohr-Rutherford diagrams are simple atomic models that show the number of electrons in each shell of an atom. While they are a major simplification of what really happening in an atom, they can be useful to help with visualizing electrons orbiting a nucleus. Drawing Bohr-Rutherford diagrams is super easy using the following steps:

Sodium Bohr Diagram
Contents show Bohr Model of Sodium The Bohr-Rutherford model was given in 1913 after incorporating the findings of Niel Bohr in the already given Bohr model. The Bohr model is a representation of the atomic structure along with all the atomic particles in pictorial form.
Bohr Rutherford Diagram For Sodium
In atomic physics, the Bohr model or Rutherford-Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons.

sodium electron configuration Newton Desk
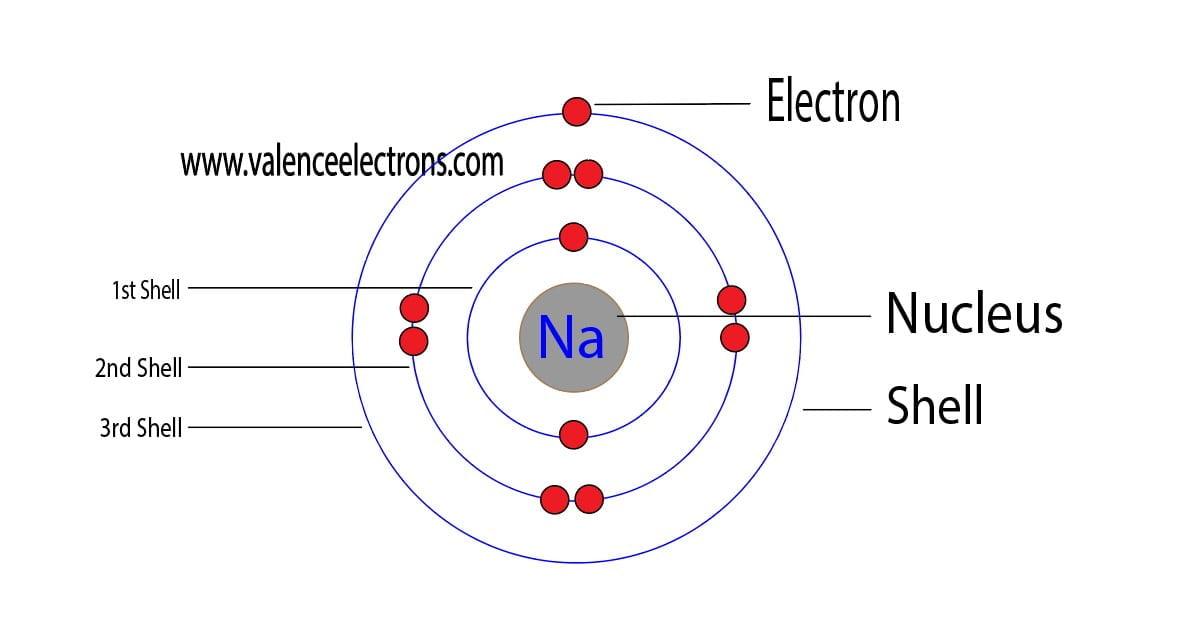
Steps Here's how you can draw the Bohr model of sodium step by step. #1 Write protons, neutrons, and electrons of sodium atom #2 Draw nucleus of sodium atom #3 Draw 1 st electron shell #4 Draw 2 nd electron shell #5 Draw 3 rd electron shell Let's break down each step in detail. #1 Write protons, neutrons, and electrons of sodium atom
[Solved] Draw a Bohrrutherford model of the ionic bond between sodium
Bohr model of Elements. 1. Hydrogen (H) 1. 2. Helium (He) 2. 3. Lithium (Li)

Draw a sketch of Bohr's model of a sodium atom. Brainly.in
.more NaCl, sodium chloride, is an IONIC compound, which means electrons are TRANSFERRED from one atom to another.You'll have to draw the Bohr-Rutherford Diagram o.

Sodium Bohr Model — Diagram, Steps To Draw Techiescientist
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.

Sodium Bohr Diagram
How to Draw the Bohr-Rutherford Diagram of Sodium chemistNATE 252K subscribers Subscribe Share 56K views 4 years ago Sodium has 2 electrons in its first shell, 8 in its second and 1 in.
