
Draw the Lewis structure of H2SO4 Chemistry Chemical Bonding and
By Biswarup Chandra Dey This article is regarding the most important acid, H2SO4 lewis structure, and its important facts. Let's start to discuss it. H2SO4 lewis structure is often known as Sulfuric acid. It is known as Oil of Vitriol. In most of the reactions in chemistry, we used sulfuric acid as a reagent. The acidity of H2SO4 is very strong.

H2SO4 là gì? AXIT SUNFURIC HÓA CHẤT CÔNG NGHIỆP QUAN TRỌNG NHẤT HIỆN
In this video we'll write the correct formula for Sulfuric acid.To write the formula for Sulfuric acid we'll use the Periodic Table, a Common Ion Table, and.

draw the Lewis dot structure of h2so4 Brainly.in
Structural Formula. H 2 SO 4. sulfuric acid

H2SO4 Lewis Structure Sulfuric Acid YouTube
Sulfuric acid is a dibasic strong acid. It means, it can release two hydrogen atoms to show acidic characteristics. Therefore, we can assume, there should be two -OH bonds in sulfuric acid molecule. Lewis structure of H 2 SO 4 Most stable lewis structure of H 2 SO 4 is shown below. Steps of drawing lewis structure of H 2 SO 4
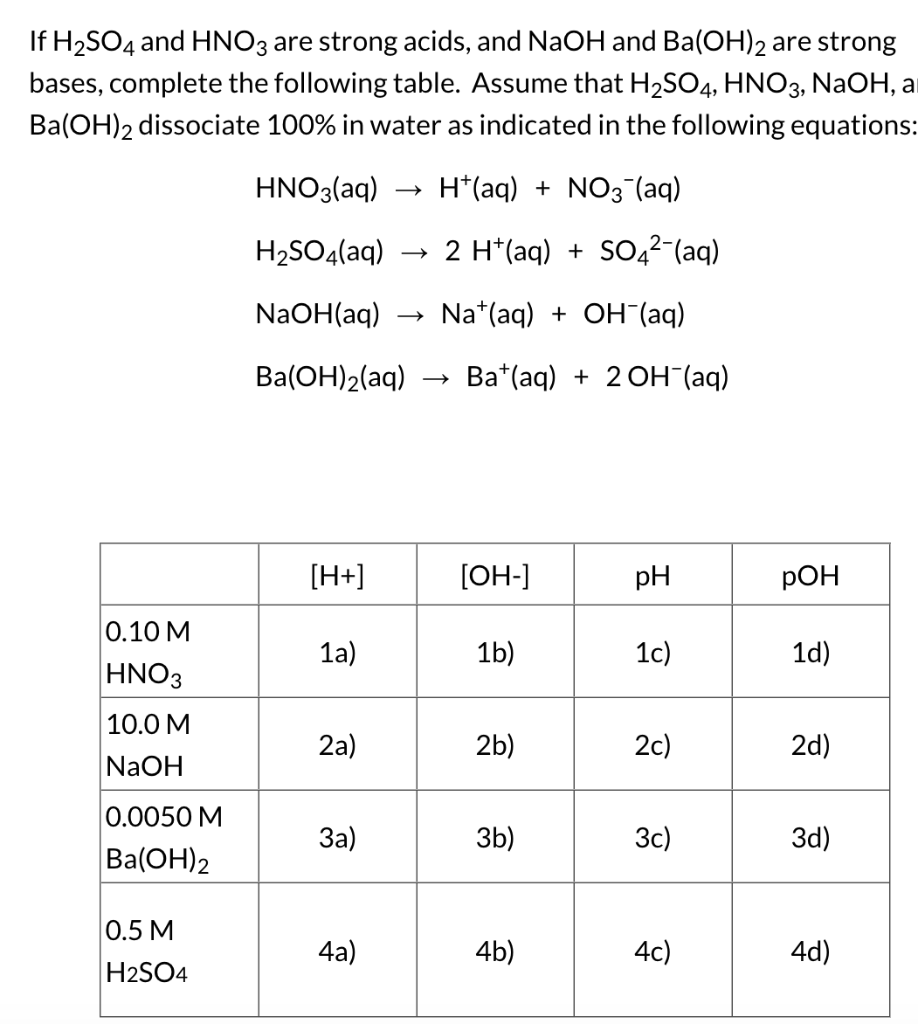
Solved If H2SO4 and HNO3 are strong acids, and NaOH and
It has a molecular weight of 98.079 g/mol. H2SO4 works as an oxidizing and dehydrating agent. Furthermore, it's diprotic in nature which holds the capacity of releasing two protons together. It is colorless, odorless, and extremely corrosive in nature.

1 molar solution of h2so4 calculation of 1 molar h2so4 1 M sulfuric
In the H2SO4 Lewis structure, there are two double bonds and two single bonds around the sulfur atom, with four oxygen atoms attached to it, and on each. For sulfur atom, formal charge = 6 - 0 - ½ (8) = +2. For top oxygen and bottom oxygen atom, formal charge = 6 - 6 - ½ (2) = -1.
H2SO4 Molecular Geometry / Shape and Bond Angles YouTube
Quantity Value Units Method Reference Comment; Δ r H°-132.3 ± 2.9: kJ/mol: RSC: Blanchard, Joly, et al., 1974: solvent: Sulphuric acid aqueous solution; The reaction enthalpy relies on -10.6 kJ/mol for the enthalpy of solution of EtOH(l) and on 35.1±0.1 for the enthalpy of solution of K2SO4(cr) Blanchard, Joly, et al., 1974.; MS
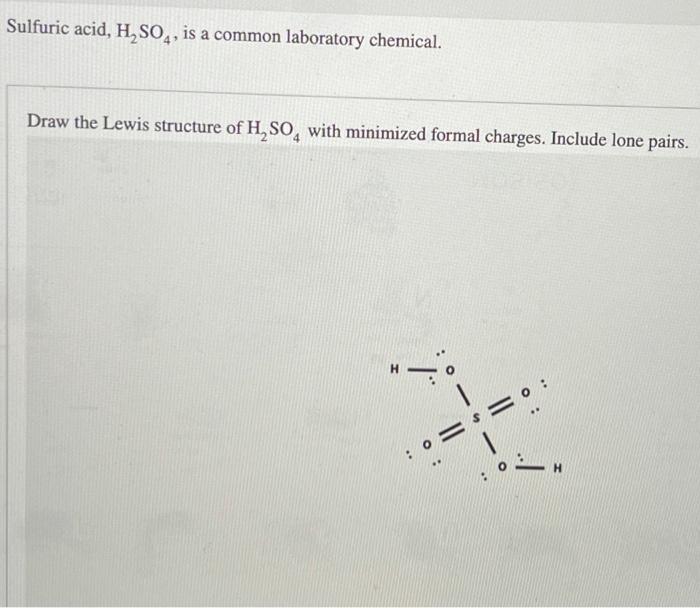
(Get Answer) Sulfuric Acid, H2SO4, Is A Common Laboratory Chemical
In order to calculate the formal charges for H2SO4 we'll use the equation:Formal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding el.
During electrolysis of H2SO4 (aq) with high charge density, H2S2O8 is
How to calculate the formal charges on H2SO4 atoms? The formal charges can be calculated using the formula given below: The formal charge of an atom = [valence electrons of an atom - non-bonding electrons - ½ (bonding electrons)] The valence electrons (V.E) of an atom are the total number of electrons present in its valence shell.
How to Balance S + H2SO4 = H2O + SO2 (Sulfur + Sulfuric acid) YouTube
Sulfuric acid is prepared industrially by the reaction of water with sulfur trioxide ( see sulfur oxide ), which in turn is made by chemical combination of sulfur dioxide and oxygen either by the contact process or the chamber process.

Solved Draw The Lewis Structure For Sulfuric Acid, H_2 SO...
In order to calculate the formal charges for HSO4 - we'll use the equation:Formal charge = [# of valence electrons] - [nonbonding val electrons] - [bonding e.
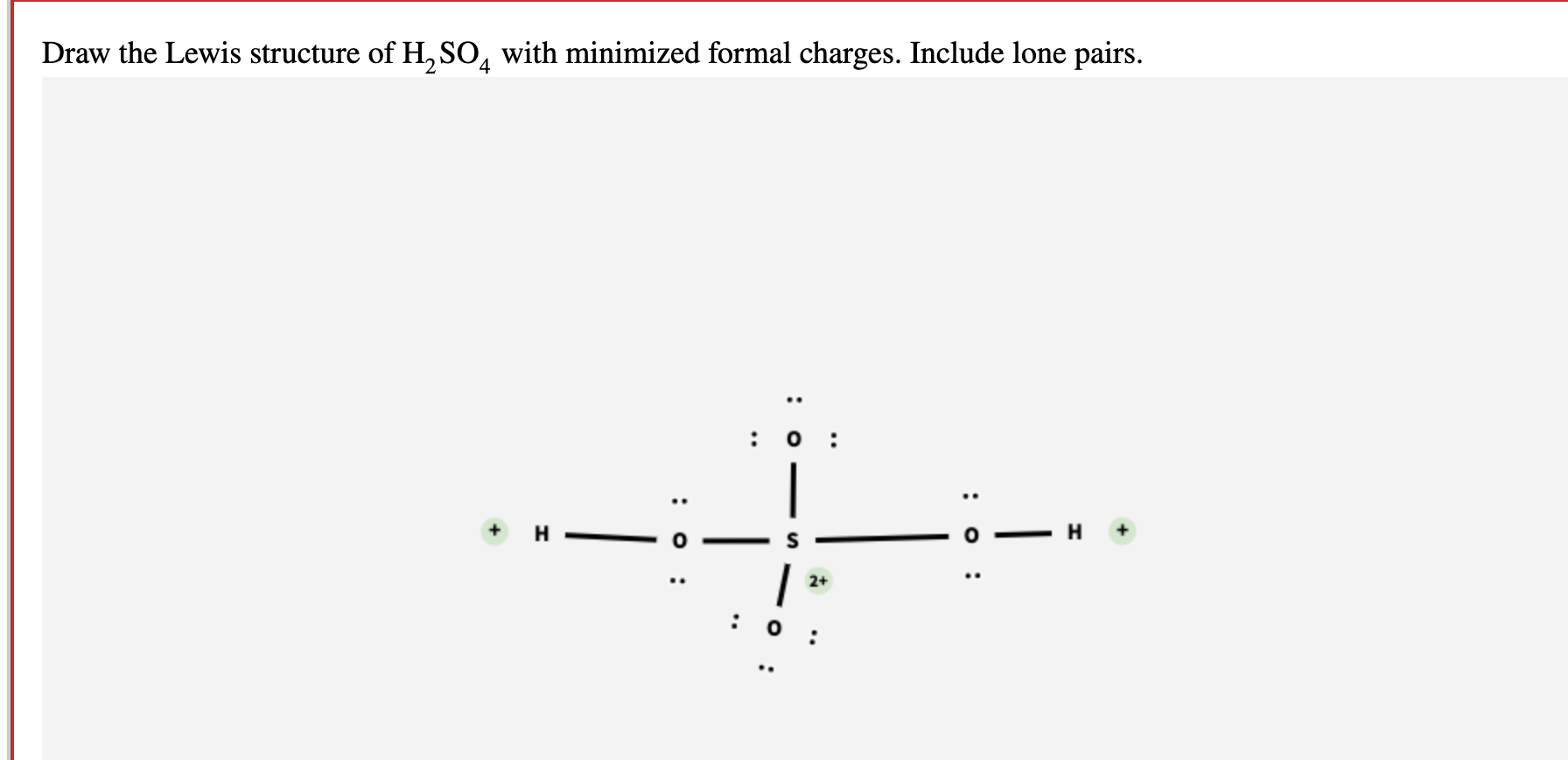
Solved Draw the Lewis structure of H2SO4 with minimized
Chemistry Basics of Chemical Bonding Question Write Lewis structure of the H 2 S O 4 and show formal charge on each atom. Solution Verified by Toppr Was this answer helpful? 0 Similar Questions Q 1 (i) Write Lewis structure of the following compound and show formal charge on each atom. H N O3 (ii)
During electrolysis of H2SO4, (aq) with high charge density. H2S2O8s is
And Why? May 26, 2023 by Jay Rana The Charge of H2SO4 (Sulfuric acid) is 0. But the question is how can you say that the charge on H2SO4 (Sulfuric acid) is 0? Well you can say this by calculating its formal charge. So let's calculate the formal charge of H2SO4 (Sulfuric acid).

H2SO4 Lewis Structure, Molecular Geometry, and Hybridization
The acid-base strength of a molecule depends strongly on its structure. The weaker the A-H or B-H+ bond, the more likely it is to dissociate to form an H+ H + ion. In addition, any factor that stabilizes the lone pair on the conjugate base favors the dissociation of H+ H +, making the conjugate acid a stronger acid.

Draw the Lewis structure of sulfuric acid H2SO4 with minimized formal
The concentration of acetic acid in the final solution will drop below 0.10 M, but the total of the two species must equal 0.10 M, the initial amount that was put into solution. [Acetic acid] + [acetate] = 0.10 M. The charge balance must account for all positively charged (sodium and hydronium ions) and negatively charged (acetate and hydroxide.
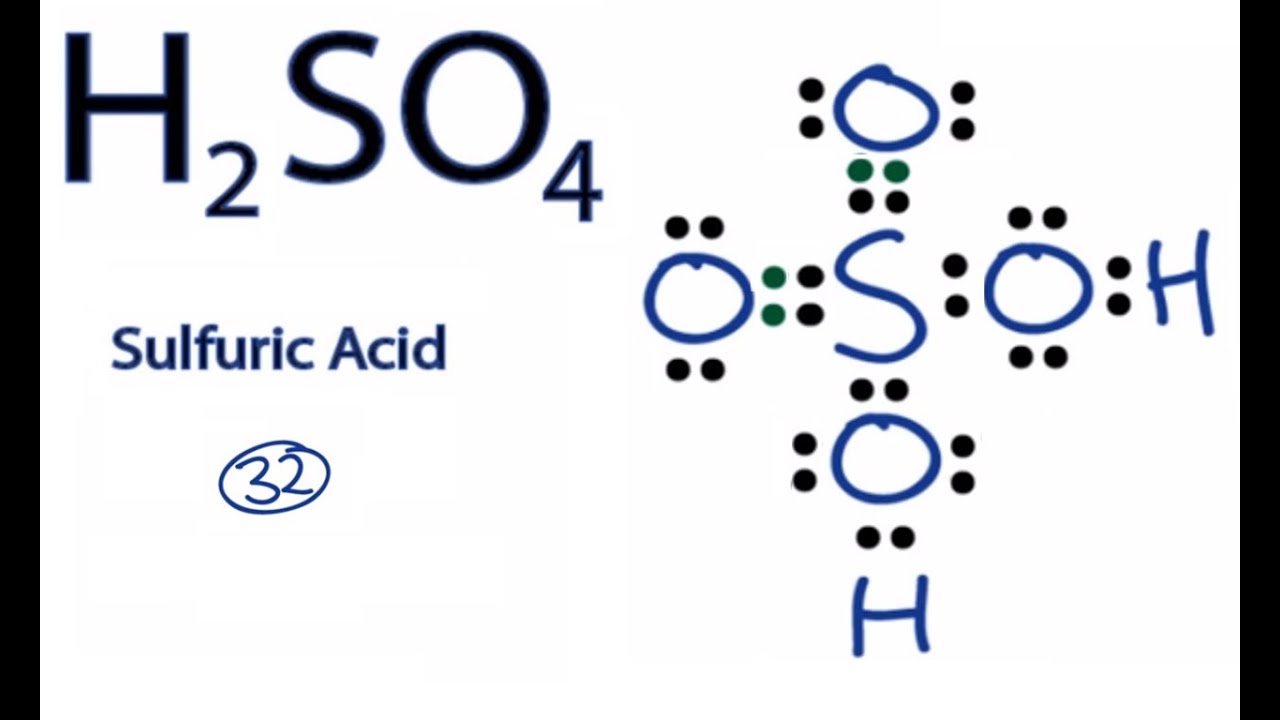
lewis dot structure for h2so4
Sulfuric acid ( American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling ), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula H2SO4. It is a colorless, odorless, and viscous liquid that is miscible with water. [6]
